The market for solvents is slowly dwindling in the developed world, due to increasing environmental and regulatory pressures—as well as concerns over toxicity to humans.
Today, 99 percent of the solvent market still is in petroleum-based materials. The list of potential problems connected with these solvents use is long and daunting—among them: inherent toxicity, flammability, explosivity, stratospheric ozone depletion, atmospheric ozone production, and global warming potential.
While there is no perfect substitute for these commercially available formulas, the search for “greener” substitutes is on, according to research results just released by Englewood, Colorado-based IHS (News - Alert), a global information company.
The report, “IHS Chemical Global Solvents Report: Opportunities for Greener Solvents,” notes that several bio-based materials are technically feasible —for example, methyl soyate, ethyl lactate and D-limonene—but have enjoyed success only in niche markets. Other novel solvents, such as ionic liquids—which are created as much in consideration of their environmental impact as of their cost and technical feasibility—are being developed.
What We Need
While there is no universal definition of a "green solvent," the report notes that one dozen principles have been suggested by Anastas and Warner, authors of “Green Chemistry: Theory and Practice ,” Oxford University Press, as follows:.
- Waste prevention–It is better to prevent waste than to treat or clean up waste after it has been created.
- Atom economy–Methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Less hazardous chemical synthesis–Whenever possible, synthetic methods should be designed to use and generate substances that possess little or no toxicity.
- Safer chemicals–Chemical products should be designed to do their desired function with minimal toxicity.
- Less hazardous solvents and auxiliaries–The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.
- Energy efficiency–Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure.
- Use of renewable feedstocks–A raw material or feedstock should be renewable rather than depletable, whenever technically and economically practicable.
- Reduction of derivatives–Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
- Catalysis–Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Planned degradation–Chemical products should be designed so that, at the end of their function, they break down into innocuous degradation products and do not persist in the environment.
- Real-time analysis for pollution prevention–Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Inherently safer chemistry for accident avoidance–The form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Current Consumption
Today, nearly half of all solvents used globally are for paints and coatings, but other uses include adhesives, inks, pharmaceuticals, chemical processing, metal and dry cleaning solutions and agriculture.
According to the report, global consumption of solvents is significant, with more than 28 million metric tons (MMT) consumed in 2012. China accounted for more than 9 MMT of that global demand, which was nearly double the 5 MMT in solvent consumption of North America. Europe consumed nearly 6 MMT of solvents last year, followed by other Asian countries (as a group) at 4 MMT, while Japan consumed just 2 MMT of solvents.
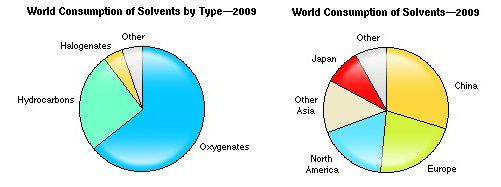
Demand for solvents in China is expected to increase approximately 5 percent to 6 percent per year during the next few years, said IHS, but solvent demand is expected to remain flat or decrease in the United States and the European Union, due to more environmental regulations; the penetration of technologies that consume little, if any solvent; and greater solvent recovery and recycling. Another factor impacting demand will be the off-shoring of industrial processes that consume large amounts of solvent to China, such as the coating of wood furniture and the assembly of shoes using solvent-borne adhesives.
Solvents are one of the most comprehensively regulated classes of chemicals, and tend to be regulated collectively as volatile organic compounds (VOCs), which contribute to lower-level ozone formation.
Edited by Blaise McNamee
 Internet Telephony Magazine
Click here to read latest issue
Internet Telephony Magazine
Click here to read latest issue CUSTOMER
CUSTOMER  Cloud Computing Magazine
Click here to read latest issue
Cloud Computing Magazine
Click here to read latest issue IoT EVOLUTION MAGAZINE
IoT EVOLUTION MAGAZINE